The (adhesive) power of mollusks
More and more, scientists are inspired by nature when solving difficult problems. Mussels stay put in turbulent tidal currents by gluing themselves to the seabed; we could do with a type of waterproof glue like that, too. Doctoral candidate Dina Ribena dove into the secret of the mussel.
For centuries, moisture has been an important factor in glue becoming unstuck. Water gradually reduces the adhesive power of natural, protein-based glues. Although modern, synthetic polymer glues are much more moisture resistant, they’re not great for gluing things together underwater under turbulent circumstances.
It’s all the more striking then, that mussels seem to have found a solution to that problem. These shellfish prefer to settle right in the middle of high and low tide – a place where powerful tidal currents pass eutrophic water through their wide-open shells. They do of course run the risk of either being washed up to the deadly shore or being dragged to the depths of stagnant waters where there’s hardly any food to be found.
Mussels can cling to a number of bases by secreting a unique mix of sticky proteins that harden into byssus threads, with which the animal can realize a rock solid attachment. That, along with their aerodynamic shape, makes them pretty much immune to even the most violent tidal currents.

What’s the mussel’s secret? How do they manage to do what we can’t, despite all our technical knowledge? Scientists and glue manufacturers would love to be able produce this wondrous sticky substance. Harvesting the product from mussels directly is too expensive. The Latvian doctoral candidate Dina Ribena tells us that thousands of mussels are needed for a single gram of pure ‘mussel glue’.
She continues to explain that it’s yet unclear how the dozens of proteins in the mussel glue work together, but we do know some of the most important ones contain the so-called DOPA molecule. “DOPA is a chemical that changes into dopamine in the body, which is a well-known neurotransmitter causing feelings of being in love, for example.”
Dopamine is easy to make and that opens up possibilities: recent research has shown that it’s possible to add a coating of dopamine to a large number of materials. Ribena: “You simply dissolve the dopamine in water and then submerge the substrate that needs coating. The dopamine forms a hydrophilic layer on which cells can be grown, for example.” And that can be used in all kinds of biomedical applications.
Ribena stresses these dopamine coatings can’t be used as actual, everyday glue – they probably can’t glue heavy things together – but a derivative of the mussel glue may very well be used as a primer onto which a second layer (the top coating, which also may consist of cells) adheres well.
It’s great dopamine can be used for this, but why it works – and how it can be improved – is barely known. Ribena’s work was supposed to shed some light on the matter. She coated several test surfaces with a number of dopamine layers (gold and sapphire, representing precious metals and metal oxides respectively). Initially she used the method described above and submerged them in a dopamine solution.
She then meticulously researched the resulting coatings using an array of techniques. “These kinds of nanolayers are not analyzed easily. You need to incorporate a wide array of measuring techniques to be able to get an accurate idea of what you’ve created.” Ribena used laser and x-ray spectroscopy as well as scanning microscopes to visualize the surface.
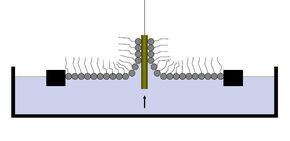
After many time-consuming measurements, she concluded the only way to learn more about the mussel glue is by studying a cleaner system. To that end, she equipped dopamine molecules with two types of long molecular tails. Those tails enables her to apply clean, single-layer coatings by means of a chemical procedure known as Langmuir-Blodgett (see box). On top of that, molecular tails easily stick to a conventional glue layer needed to measure the strength of the dopamine adhesive.
To one of the two dopamine versions an amide group was added, the other remained free of additives. The amide group turned out to create hydrogen bridges – flexible interconnections between hydrogen atoms of two tails. This process makes for a cleaner single layer, which is exactly what you want in a primer. “The version without amide group caused much more irregularities, and that’s important to know because it affects the power of the glue.”
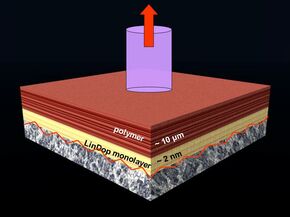
The mechanically coated substrates have their molecules neatly positioned in the same direction, with the dopamine side stuck onto the substrate’s surface and the tails facing away from it. “I applied a top coating to that, which makes cross links with the tails of the dopamine molecules.” Finally, she glued a small metal rod onto the top coating using an epoxy adhesive for the ‘pull-off test’. “In this setup, the attachment of the dopamine to the substrate is the weakest link. By measuring the exact force needed to release the rod, the adhesive power of the dopamine glue can be determined.”
The doctoral candidate says many researchers don’t bother to subject their coatings to realistic tests. To be able to say anything at all about the adhesive power, you’ll have to expand the system – pulling apart separate molecules using a scanning microscope doesn’t say much about what happens on a macroscopic level. “Only a small number of researchers have provided realistic data.”
Ribena’s tests have shown that her mussel-glue-based primer has three times more adhesive power compared to untreated metal, meaning the top coating sticks to the primer three times better. “My tests were based on those of the industry; they’re not entirely scientific, but the results are good. I’m fairly confident my study will see a follow-up.” That last bit is what matters to her most: “My dream is that this research will live.”


Discussion