- Research , Student
- 29/05/2015
New student team wants to build first-ever car on formic acid
FAST, a new team of TU/e students, wants to have built the very first formic acid-fueled car within a year. The students continue Georgy Filonenko’s Ph.D. research, who graduated with honors back in April. They hope to travel Europe in their formic acid car in the summer of 2016.
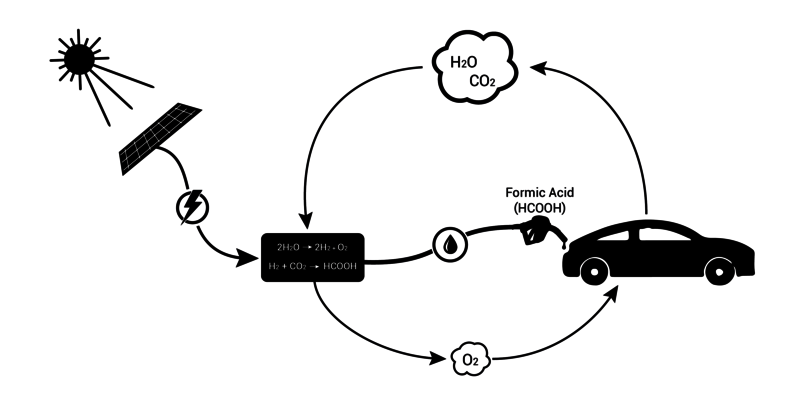
Hydrogen is considered the energy carrier of the future. But the gas can only be stored and transported under high pressure, which makes the use in vehicles both challenging and dangerous. Adding carbon dioxide (CO2), water can be converted to formic acid and vice versa: less risky to transport and store.
The downside is that the process is quite slow. During research, TU/e PhD graduate Filonenko discovered – partly thanks to two students of Chemical Engineering – a catalyst that speeds up the reaction immensely, and was able to optimize it, too.
Students of the Energy Transition honors track realized the technology offered opportunities and challenges, and combined their powers in team FAST (Formic Acid Sustainable Transportation). Their goal: building a real car that runs on formic acid.
One of the benefits of a car that runs on formic acid compared to an electric car, for example, is the longer distance it can travel without refueling. And refueling is much less time-consuming than electric charging, says team member Tim van Lohuizen (Industrial Engineering).
The students want to build a car with “a fair range. Some standard cars can travel up to one thousand kilometers on a single tank. I’d be happy is our car got three to four hundred kilometers”, says Van Lohuizen.
The plan and timeframe are ambitious but doable, according to the students. If the team, currently eleven strong, finds a sponsored hydrogen car they can turn into a car that runs on formic acid, that is. Max Aerts (student of Industrial Design), FAST member: “There are a bunch of prototypes out there, but manufacturers claim the market is not ready.”
One of the major challenges FAST will be facing next year according to chemistry student Mitch Winkens, is separating the CO2 and hydrogen that is released from formic acid: “These are two gases that you want separated properly. If you don’t, the fuel cells that use the hydrogen become clogged due to CO2 that’s still present”.
Another serious is ‘upgrading’ the technology. Sure, it’s been successful on a small scale (the students have managed to have a tiny car run on formic acid, however slowly), “but we need a bigger reactor. It will involve quite some control systems and we’re not sure how to handle those yet”, says Van Lohuizen.
The students hope to have their ‘formicar’ on the road by the summer of 2016. They’d like to visit a number of European universities where they can refuel formic acid produced at those very universities.
Discussion