Injectable ‘chemo gel’ is possible alternative to abdominal rinses in abdominal cancer
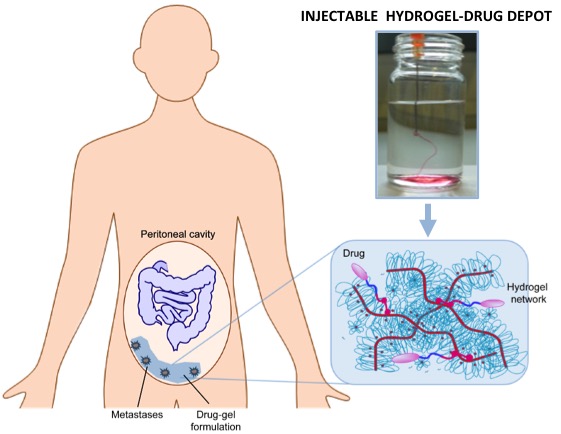
Abdominal cancer is one of the deadliest types of cancer. Without treatment, the expected survival is about 6 to 12 months. A gel that can be injected into the abdominal cavity to deliver very local chemotherapy for weeks, could be a better alternative to the chemo abdominal rinses that are common today. Professor Patricia Dankers of Eindhoven University of Technology (TU/e), who stood at the basis of this hydrogel, will now test this gel in a four-year preclinical study together with researchers from Maastricht UMC+ and Catharina Hospital. The Dutch KWF Cancer Society is backing this research to the tune of almost 600,000 euro.
The developed hydrogel is programmed in such a way that it is liquid during injection, but becomes more solid after it is released into the body. The gel is filled with a widely used anticancer drug (better known as chemo), which is released over a period of days or even weeks. Dankers: “This new treatment combines the advantage of local chemotherapy with a non-invasive, long-term administration method. Patients who are not eligible for current forms of treatment are therefore likely to be able to receive treatment using this technique.”
Severe current treatment
Abdominal cancer, also called peritoneal cancer, is mainly caused by metastases, or spreading, of intestinal cancer. One in eight patients with bowel cancer develops metastases in the abdominal cavity. At the moment, the only promising treatment is an operation, in which the metastases are surgically removed, combined with a flush of local chemotherapy. In this local treatment, the abdominal cavity is rinsed for 90 minutes with chemo (so-called Hypertherme Intra Peritoneal Chemotherapy or HIPEC). Ignace de Hingh of Catharina Hospital: “The effect of this flush is limited because of its short duration. In addition, a large group of patients (1 in 4) is not eligible for this treatment because they are considered insufficiently fit to undergo this severe treatment. The effect then does not sufficiently outweigh the distress suffered by the patient.”
Illuminating tumor cells
The preclinical rat study that the consortium will now carry out consists of a series of experiments to investigate the safety and efficacy of the chemo gel. “For this we use tumor cells that we make visible by illuminating them so that a special camera can externally, and based on the intensity of the light, determine how many tumor cells are present in the abdomen and thus how successful the treatment is,” says Nicole Bouvy of Maastricht UMC+. For example, the tests should show whether the combination of the gel with the anticancer agent is safe, whether the gel works just as well or perhaps better than the rinse, and whether the gel is still successful after surgery. The extensive laboratory study has now been completed and shows promising results. If the preclinical study proves successful, the researchers will be one step closer to the clinical application for patients.
A research grant from the KWF has been awarded to a consortium of Maastricht UMC+, Catharina Hospital and Eindhoven University of Technology to develop a new strategy to improve intraperitoneal chemotherapy for patients with peritoneal cancer. Within this consortium, Professor Nicole Bouvy (UMC), Professor Ignace de Hingh (Catharina Hospital) and Professor Patricia Dankers (TU/e) are joining forces to initiate preclinical validation of an innovative supramolecular hydrogel that enables continuous local intraperitoneal chemotherapy.


Discussion