Pig heart or hybrid heart, which is worth our time?
Postdoctoral researcher Marijn Peters on the future of heart transplants
In the Netherlands – as well as worldwide – there’s a great shortage of donors. Particularly donor hearts are very scarce. To reduce waiting lists, intensive research into alternatives is being conducted. Two such alternatives are the pig heart and the hybrid heart. Cursor talked all of this over with Marijn Peters, postdoctoral researcher at the TU/e Soft Tissue Engineering and Mechanobiology research group and the Regenerative Medicine Center of Wilhelmina Children’s Hospital in Utrecht.
Last October, an American man who had gotten a modified pig heart ended up dying six weeks after the surgical procedure. 58-year-old Lawrence Faucette was the second person ever to receive such a genetically modified pig heart as a donor organ. The first patient, 57-year-old David Bennett, had died eight weeks after the transplant.
Is there any hope for the pig heart?
“What’s important to remember about these patients is that they were terminally ill. They only carry out these kinds of studies if a patient’s chances of survival are pretty much zero,” says Peters. “The period that someone with such an organ can live for is getting longer. The most recent person survived for almost six weeks, eventually dying of something that may have been preventable. So there’s still hope it will work in future.”
Both patients that received a pig heart ended up dying from a pig virus, the porcine cytomegalovirus. A variant of the cytomegalovirus also occurs in people and is generally innocent. That’s why Peters doesn’t think the virus in itself was the issue, but rather that it was being suppressed in the pig but not in the human recipient. In combination with heavy immunosuppressants, drugs that someone gets after receiving a donor organ, this is likely to have caused the problems. Immunosuppressants make sure that the recipient’s immune system is suppressed so the foreign donor organ isn’t rejected. “It’s very difficult to guarantee with absolute certainty that a donor organ doesn’t have any pathogens (organisms that cause disease, ed.) anymore,” Peters indicates. The problem with heavy immunosuppressants, as they are currently being administered, is that if there are any viruses present they can quickly lead to death. Even if the virus isn’t serious at all.”
She emphasizes that even if a donor organ is completely free of viruses, a suppressed immune system still entails risks. “The patient can of course also be infected with a virus from the outside, such as the flue or coronavirus. If the recipient’s immune system is suppressed to such an extent, it makes it very difficult for the body to fend off the virus. What it comes down to is that all human beings need well-functioning immune systems.”
Risks aside, immunosuppressants play an important part in the integration of the new donor organ into the recipient’s body and are currently an essential part of the transplant process, Peters says. She therefore thinks a new balance is needed when it comes to immunosuppressants. “You want the human immune system (of the recipient, ed.) to function in such a way that it doesn’t cause rejection, but does continue to do its work. That balance isn’t always there at the moment.”
Virus as a solution
To create this balance, Peters researches the possibility of reducing or even preventing the strong immune response following a transplant, thus decreasing the risk of rejection. If this can be applied successfully, an added bonus would be the decreased need for immunosuppressants.
To make this happen, viruses are used. This may sound like a paradox, but Peters explains why viruses are actually needed in this respect. “Viruses are very efficient. They are very well-protected against the host’s immune system so they can go unnoticed,” she says. “If we can use this quality to make donor organs appear as natural parts of the body, patients may need way fewer immunosuppressants.”
A specific part of viruses is used to this end: the US2 protein, which is responsible for ‘evading’ the immune system. “This protein prevents the placing of what looks like little flags on the outside of the donor cells, which are normally used by the immune system to recognize foreign cells,” Peters explains. “If these flags don’t appear – or appear to a lesser extent – on the outside of the cells, this means the immune system can’t recognize the donor cells and there’s a smaller chance of them being rejected.” The official jargon for these flags is human leukocyte antigens, or HLAs.
The insertion of the US2 protein is done by means of transduction. The gene for the protein is packaged into a carrier virus that is unable to propagate. As a result, the virus is not harmful but does still have the capacity to invade a human cell. Once there, the gene can be expressed to produce the US2 protein and enable it to prevent the ‘little flags’.
The research described above is carried out by TU/e in collaboration with Leiden University Medical Center (LUMC). LUMC already demonstrated the principle by transplanting human alveolar cells in mice. “They already showed it can be done with cells,” Peters says. And now we (at TU/e, ed.) are looking into using that system for entire tissues and organs, through the transplant of human heart valves.”
The hybrid heart
This technique wouldn’t only increase the chance of success for pig heart transplants, but also for other alternatives, such as the hybrid heart. Just like donor hearts originating from people or pigs, a hybrid heart could be transplanted as a donor organ to take over the entire heart function.
Previous systems have never been able to do so. Take the Left Ventricular Assist Device (LVAD), a system that’s hooked up to a patient’s own heart. As its name suggests, an LVAD doesn’t take over the heart’s entire function but only supports the left ventricle, which is the heart chamber that pumps oxygen-rich blood to the rest of the body. As an LVAD doesn’t take over the entire heart function, it can only be used as an intermediate solution for people waiting for a donor heart, for instance.
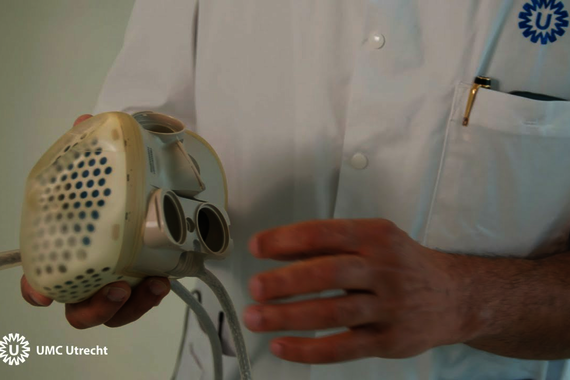
While an LVAD can offer support and buy time, it doesn’t provide a long-term solution. This created demand for an artificial heart that can be used as a full-fledged donor organ. This is often called a ‘hybrid heart’, because most artificial hearts also contain biological material and are therefore partly mechanical, partly biological. “It’s a mechanical system, an artificial heart, which is humanized to a certain degree,” Peters adds.
In 2021, the first-ever Dutch person received a hybrid heart. This hybrid heart consisted of an artificial shell made of polyurethane, lined on the inside with biological material originating from the pericardium of a bovine. Using a cable running through the abdominal wall, the hybrid heart was hooked up to a controller and a set of batteries. The patient could carry the controller, weighing about three kilos, with him in a shoulder bag. The patient was a 54-year-old male who wasn’t eligible for a regular (human) donor heart because of severe heart failure and poor health. He went on to live for over eight months with his hybrid heart before passing away in June of 2022.
The aforementioned version of the hybrid heart is just one of the possible examples. Several alternatives and variants are collectively known as ‘hybrid heart’; this list is likely to continue growing.
In Eindhoven, work is being done on a hybrid heart with specific human tissue. This would reduce the risk of rejection, thereby increasing the chance of survival. The Hybrid Heart Consortium, which the TU/e Soft Tissue Biomechanics and Tissue Engineering (STBE) research group is part of, is conducting research in this field.
There are different ways of humanizing an artificial heart. It can be lined with human donor tissue, but it’s even safer to line it with the patient’s own tissue. “Patricia Dankers’ group is currently working on a type of hydrogel,” says Peters. This lines the inside of the hybrid heart. When the heart is transplanted into the recipient, this gel forms a layer to which the patient’s own circulating cells can attach.”
Peters explains that this technique is based on the principles we see in wound healing. “When you get a wound, all kinds of repair processes are activated to create new tissue. The idea is to take the mechanism of relining a wound and use it on such a heart,” she says. “That way you can make a heart that’s half artificial, half the patient’s own.”
Which is worth our time?
So at the moment, in addition to traditional, person-to-person transplants, the focus is on two main alternatives to mitigate donor shortages. Xenotransplants, i.e. transplanting an organ of non-human origins such as a pig heart, and more mechanical systems that are being humanized, such as the hybrid heart. But which of the two should science concentrate on? “The problem is that throughout the research field, we are trying to minimize the use of animals,” says Peters. “So if you then develop a whole system with pigs serving as a donor depot for people, this raises major ethical questions.”
After all, pigs whose hearts can serve as donor organs are specifically bred and genetically modified for this purpose. This includes adding a number of human genes and silencing a few pig genes. The pigs are also periodically screened for all kinds of pathogens.
The involved TU/e research groups have therefore chosen to focus on the second alternative: the hybrid heart. “Why would we invest all of our time and means into making an animal organ depot?” Peters wonders out loud. “We’re so good at tissue engineering now. We’ve made loads of progress on creating a hybrid heart that may work equally well.” Even though the focus is on alternatives closer to humans, research in Eindhoven may also yield valuable knowledge for the future of xenotransplants.


Discussion