Lab-made blood vessels: mechanics matters
Eline van Haaften, PhD candidate from Biomedical Engineering, developed a bioreactor that mimics blood circulation within the body. She identified some of the possible causes of failure of lab-made vessels. Her results could be used to improve the design of lab-made vessels and accelerate clinical translation for patients with cardiovascular and kidney diseases. Van Haaften defended her PhD thesis on Tuesday October 1st and was rewarded with the honor of 'cum laude'.
Traditional interventions to cure patients with diseased blood vessels require the transplantation of patients’ own or donors’ arteries or veins. This generates an enormous clinical demand for blood vessels availability. To alleviate the shortage of blood vessels, synthetic vessels are often implanted. However, these implants carry a greater risk of blood clots and infections. Also, they cannot adapt while the patient develops and ages.
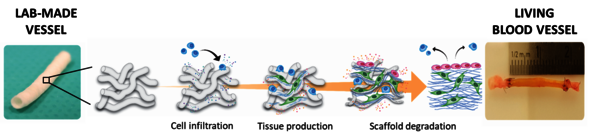
Lab-made blood vessels might be a game changer for millions of patients with cardiovascular diseases. For Eline van Haaften, PhD candidate in the group of Soft Tissue Engineering and Mechanobiology led by prof. Carlijn Bouten, 'it all starts with a cell-free, biodegradable material' shaped in the form of a blood vessel (‘scaffold’). “Once implanted in the body”, explains van Haaften, “this scaffold can recruit patient’s own cells and promote the formation of new vascular tissue”.
As new tissue is deposited, the scaffold slowly disappears, leaving behind a completely new and fully functional blood vessel. Scaffolds in different diameters and lengths can be easily produced, and stored in massive amounts in the lab or even directly in hospitals. Also, because they lack biological components, the risk of rejection is practically nonexistent.
Easy to make, cheap and safe. What else, one might wonder. Yet, as soon as the scaffolds are implanted, the challenge starts. The scaffolds need to be well adapted to specific mechanical demands arising from blood flow and pressure. Also, the porous structure of these scaffolds is instantly colonized by cells of the immune system. These cells can sense and respond to the mechanical loads imposed by the blood flow. The stability of the newly deposited vascular tissue depends on how these cells respond to these mechanical loads. So does the outcome of the implantation.
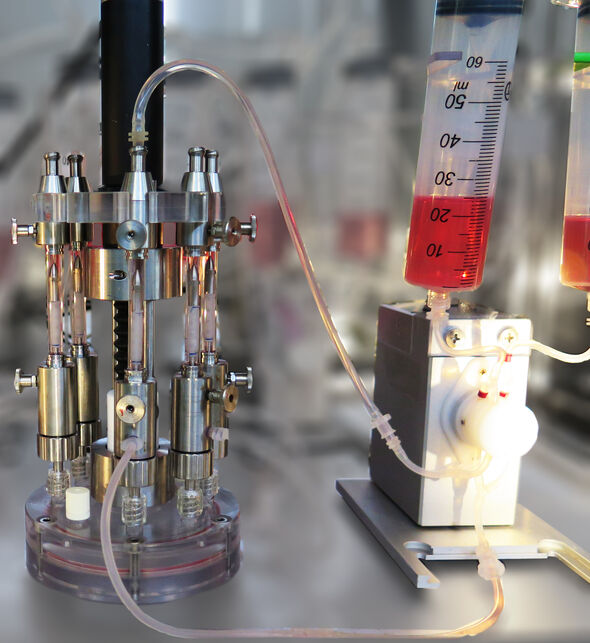
To understand how cells respond to mechanical loads, Van Haaften developed a bioreactor that can recreate blood flow and pressure. She seeded fibrous scaffolds with vascular cells, and exposed them within the bioreactor to mechanical loads arising either from the blood pressure (stretch), the blood flow (shear stress), or a combination of the two.
She demonstrated that when the implanted vessels are stretched by blood pressure, their structure degrades at a slower pace and the deposition of new vascular tissue is favored. Also, she demonstrated that shear stress occurring at the walls of the synthetic vessels as blood flows limits excessive deposition of tissue.
Van Haaften: “These results underline the importance of balancing the mechanical loads arising by both blood flow and pressure. As we need the synthetic vessel to degrade while the living-like one is formed, the synthetic vessels should be pliable enough when exposed to blood pressure. We can achieve this by, for example, adjusting the thickness of the implanted vessels. Yet, we also need the cells infiltrating the vessels to experience shear stress as blood flows, so that the newly formed tissue is remodeled. To do so, we can vary, for example, the vessel diameter and its level of porosity.”
Beyond cardiovascular diseases
“These synthetic blood vessels”, Van Haaften explains, “can be used also in patients undergoing dialysis for kidney diseases”. Right now, the canonical way to give patients dialysis is by creating a fistula, a surgically-made link which connects a vein and artery in the patient's arm or leg. The fistula allows blood to travel through tubes straight into the dialysis machine, where toxins are removed. Yet, some patients' veins are just not strong enough.
Lab-made vessels might once again be the way to go. Yet, they often fail due to abnormal narrowing of their diameter (stenosis). This stricture is caused by excessive proliferation of cells and abnormal deposition of tissue, which progressively closes up the inner diameter and blocks the blood flow. Yet, the exact mechanisms by which this occurs remains unclear. In this case, Van Haaften discovered that abnormal oscillations in the blood flow - possibly happening when the diameter of the implanted vessel is different from the native vessel - can activate abnormal cell proliferation and tissue deposition.
UNESCO rising talent
These results can be used to better design lab-made vessels and get one step closer to their safe applications in patients with cardiovascular and kidney diseases. For her research, Van Haaften received an honorary mention from the L’Oreal-UNESCO Rising Talent Prize for women in science; an initiative to promote the academic careers of young female researchers in the Netherlands.
Van Haaften is not new to academic distinctions. Both her BSc and MSc diplomas were awarded 'cum laude'.
![[Translate to English:]](/fileadmin/_processed_/0/c/csm_elinevanhaaften-bioreactor_16dba9be8e.jpg)


Discussion