Home Stretch | Sustainable hydrogen sourced from biogas
In the future sustainable sources will have to provide not only our energy but also our raw materials. This could mean, for example, extracting hydrogen from biogas. Doctoral candidate Niek de Nooijer showed that with use of sophisticated membrane reactors this is a real possibility.
Hydrogen is not only regarded as one of the most important energy carriers in the sustainable economy of the future, it is already an essential raw material in a wide range of chemical and industrial processes. The problem, however, is that hydrogen is currently extracted mainly from natural gas, which we would rather leave in the ground. This prompted process engineer Niek de Nooijer to research whether hydrogen can also be extracted from biogas.
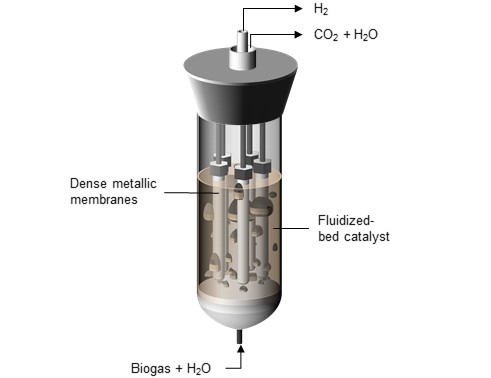
Most hydrogen is currently produced by mixing natural gas (primarily methane, CH4) at a high temperature with water vapor (H2O). This gives rise to hydrogen (H2) and carbon dioxide (CO2). But this process, called steam reforming, uses a lot of energy. In view of this, work is underway at TU/e on ‘smart’ reactors, in which during the reaction the hydrogen produced is removed continuously using a membrane. As a result, steam reforming can be carried out at a lower temperature, and considerably less energy is needed.
In principle, hydrogen can also be extracted from biogas, explains De Nooijer. The fermentation of substances such as manure, sewage sludge and household green waste gives rise to biogas. “The main difference with natural gas is that biogas consists largely of CO2, which has to be removed in advance of conventional steam reforming.” Because this step alone takes a great deal of energy, biogas is not yet a logical source of hydrogen. The doctoral candidate showed, however, that the CO2 component of biogas poses no problem for a membrane reactor.
De Nooijer used a special membrane, a thin layer of palladium and silver through which only hydrogen can pass, to extract the hydrogen during the steam reforming process. In addition he uses a 'fluidized bed' as it is called, in which the catalyst (the substance that helps release the hydrogen atoms from methane) consists of rhodium particles suspended in the gas stream. “Owing to the vortices in the gas, the hydrogen is guided to the membrane much more efficiently,” De Nooijer explains.
But one challenge remains. The small amount of hydrogen sulfide (H2S) in biogas is harmful to the catalyst, and even more so to the membrane. “The sulfur atoms are drawn to the spot where hydrogen should be binding to the membrane. So this becomes, as it were, clogged. We tried to solve this by adding gold particles to the membrane, but unfortunately this didn't help enough.” As long as no suitable membrane exists, one that is not harmed by hydrogen sulfide, it will be necessary to remove the hydrogen sulfide beforehand. “By the way, this also applies to natural gas, since that also contains hydrogen sulfide,” De Nooijer points out.
An analysis conducted by the doctoral candidate reveals that hydrogen extracted from biogas using a fluidized bed membrane reactor is no more expensive than the conventional steam reforming of biogas. This is because while the membranes are still pretty expensive, less biogas is needed for the same yield of hydrogen. And the membranes will become cheaper as soon as they are produced on a larger scale, De Nooijer expects. Nonetheless, biogas - which incidentally is currently a fair bit more expensive than natural gas - is not the ultimate solution for the production of hydrogen, he stresses. “There is simply too little biogas to meet the entire demand for hydrogen, but it can certainly be a piece of the puzzle. This is a good method for producing hydrogen from biogas locally on a relatively small scale - for example, on a farm or at a water purification plant.”
![[Translate to English:]](/fileadmin/_processed_/b/2/csm_BvOF_sluitstuk_Niek_de_Nooijer_04d0a228d6.jpg)

Discussion