Home Stretch | From nurse to PhD engineer
She left Indonesia as a nurse headed for the United States. Her exceptional talent for math was discovered in Houston, and in the Netherlands she retrained as an engineer. As a doctoral candidate at Chemical Engineering & Chemistry Ria Sijabat went on to take mathematical models for electrolysis in membrane reactors to a higher level.
That Ria Sijabat will be entitled to call herself a doctor of chemical engineering at the end of this month is a minor miracle. Or at least an extraordinary story. Born in a small village on Sumatra, she started training as a nurse at the age of fifteen. Later, after the devastating tsunami, she was sent by her employer International SOS in January 2005 to provide emergency help. “Emergency service workers from all over the world were there, but it was the professionalism and equipment of the Americans that impressed me most,” she explains. “I thought at the time that if I wanted to continue to develop as a nurse, that's where I would need to go.”
Already used to being top of her class, Sijabat managed to gain a scholarship from the Indonesian government to continue her studies in the United States. Having gained her United States license as a Registered Nurse, she started work at a hospital in Houston. “But as I was working three 12-hour shifts a week, I had enough leisure time to take math courses. It was a subject I had always enjoyed.”
Engineers
She soon proved so good that she was able to help her fellow students - who were brushing up their math in preparation for an engineering study - with the arithmetic. “In Indonesia I had already become fascinated with engineering when I was sent on secondment to a gold mine. Thanks to the oil industry, chemical engineering is very important in Houston, and a professor encouraged me to take up the subject because I was so good at math.”
By now she was married to a Dutch citizen, and in 2011 this paved the way for a new study opportunity: training to become a chemical engineer at Avans University of Applied Sciences in Breda - a step that would not have been possible in Indonesia and would have been difficult in the United States because her resident's permit was linked to her work in the hospital. This study whetted her appetite and in 2016 she graduated cum laude at TU/e's Department of Chemical Engineering and Chemistry, where she was then asked to start a doctoral project. “I wanted to do something with a lot of math, but also involving a company so I gain practical experience,” this Indonesian woman tells us in excellent Dutch. Cooperation with Nouryon, formerly the chemical branch of AkzoNobel, offered her this combination.
Electrolysis
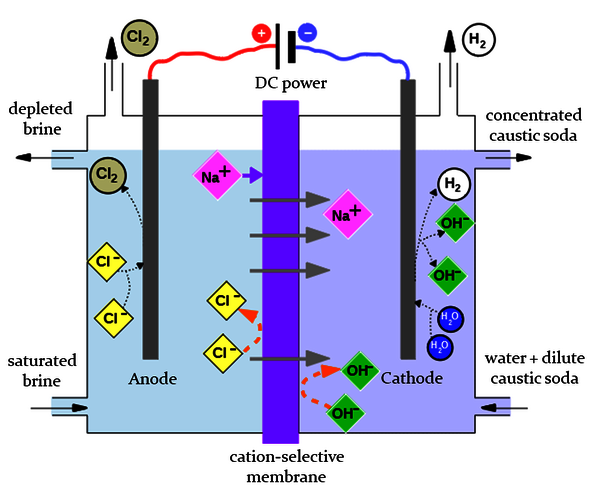
For her doctoral work, she studied the membranes used in electrolysis, a process in which an electrical current is passed through a solution in order to split substances. The electrolysis of brine to produce chlorine and caustic soda, for example, is a huge industry worldwide, Sijabat points out. Hydrogen is also released during this process, formerly a waste product but now regarded as an important sustainable energy carrier - provided, of course, that the electricity used is generated sustainably. This proviso makes splitting water directly into its constituents oxygen and hydrogen, which may be done using wind turbines at sea, another interesting proposition.
Leaving scope for this sustainable application of electrolysis, flexibility becomes an important feature of the process, so that it can be geared to the availability of renewable electricity. In which case, the membranes in the electrolyzer - which, in short, must allow every single positively charged ion to pass through while remaining impenetrable to negative ions - must be capable of withstanding a sudden increase in the current if the wind gets up or the sun comes out. “If this kind of membrane becomes too hot and is punctured, there's the risk of an explosion because the hydrogen may come into contact with chlorine or oxygen.”
Minireactor
Partly for reasons of safety, it took no less than four years to get a test reactor in the lab at TU/e in working order. “I wanted to use it to test the suitability of commercial membranes to accommodate higher electrical currents. We began with a tiny electrolyzer measuring one square centimeter - guaranteed safe at that size.” Right when the larger version was ready the laboratories closed due to the corona pandemic. “So I had no choice but to reimmerse myself in my mathematical models,” Sijabat explains. “Besides, my supervisors requested that I did so because it is my biggest strength. Leave the planned experiments to your successor to do, they said.”
Thus these mathematical models form the core of her thesis. “As it is not only impractical but also dangerous to test the membranes under every conceivable condition, you need models to predict which type of membrane performs best under which conditions.”
High concentrations
Simple mathematical models were already available for membrane electrolyzers, but these are actually only valid for highly simplified situations and for very low concentrations of the substances in solution. In the electrolyzer, by contrast, these concentrations are very high. “This is why I used the Maxwell-Stefan equations, which provide for the aspects I was working with,” Sijabat explains. No one had ever succeeded in producing a sensible model based on this approach, she says. “But I managed to make a major step forward. The model still needs to refinement but my successors can build on my work.”
While the corona pandemic did pose some problems for her at the tail end of her doctoral work, there is an upside to all the inconvenience it has caused. Her defense of her thesis will be hybrid, so her opponents and some of her guests will attend remotely. “Happily this means that family and friends from Indonesia, the United States, Japan and Columbia can also experience the ceremony!”

Discussion