Home Stretch | Better understanding the bone
In human bones, tissue is constantly being resorbed and formed by two types of cells: osteoclasts and osteoblasts. Stefan Remmers, PhD candidate at the Department of Biomedical Engineering, used human cells to cultivate a 3D model of osteoblasts and osteoclasts, which allows for visualization and quantification of both bone formation and resorption over time. In the future, the 3D model may help in the development of new treatments against bone diseases, such as osteoporosis. Remmers will defend his thesis on Tuesday, March 14.
The human bone may look like a static organ that doesn’t change much after it’s fully grown, but nothing could be further from the truth. Old and damaged tissue is constantly being resorbed and replaced by new bone tissue. Two types of bone cells are involved in these processes: osteoclasts are responsible for bone resorption and osteoblasts for forming new tissue. In human adults these two activities are in balance, but when this balance is disturbed, it can lead to degenerative diseases.
Osteoporosis
With osteoporosis, the osteoclasts are more active than the osteoblasts, which means more bone is being resorbed than formed. As a result, bone mass decreases, to the point where bone density is so low the bones become fragile and break easily. In the Netherlands, around 800,000 people – mostly post-menopausal women – have osteoporosis. “We can use drugs to slow down the decline of bone strength, but we can’t reverse the process altogether. Once you have osteoporosis, you’ll never be rid of it again,” says Remmers. “Unfortunately, we don’t know enough about the bone to cure these kinds of diseases.”
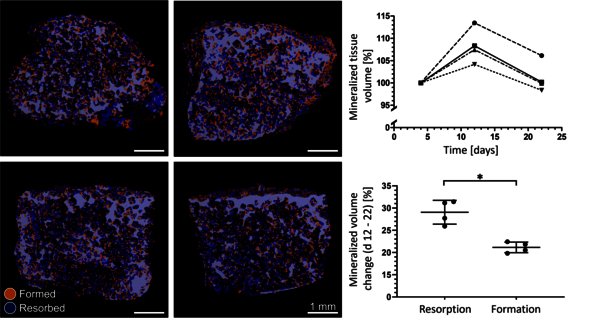
To be able to better study the complex processes of bone forming and resorption, Remmers developed a 3D model of osteoblasts and osteoclasts during his PhD. “They look like miniature bones, which measure five by three millimeters and were cultivated from human cells in the lab,” he explains. The 3D model contains both types of human cells and can therefore mimic bone forming and resorption outside of the human body. What’s special about this model, is that it allows for these processes to be quantified and visualized over time. “To be honest, I wasn’t sure if it could be done before I started,” the PhD candidate admits. Remmers’ model of osteoblasts and osteoclasts provides more insight into the complex processes involving these cells. In the future, the model may help in the development of new treatments against bone diseases, such as osteoporosis.
Unpredictable osteoclasts
“Osteoblasts are fairly easy cells to work with,” Remmers tells us. “But osteoclasts we don’t fully understand yet.” Osteoclasts are interesting cells because they don’t differentiate from stem cells as cells usually do, but are produced by cell fusion, where multiple cells combine to become one. “Sometimes it’s ten and sometimes it’s fifteen, and we don’t know why,” Remmers says. This makes the cells unpredictable.
Remmers used an existing 3D model containing osteoblasts as a starting point, but the big challenge was to add osteoclasts. What’s more, they had to be functional enough for their functionality to be measured with a CT scan. “Setting up a cell culture model is not an easy task in itself, but it’s even harder when it involves two types of cells,” the PhD candidate explains.
Remmers soon realized that he couldn’t just run some experiments in a lab. That’s why he started looking for all co-culture studies – experiments involving both osteoblasts and osteoclasts – ever published, collecting all data that could aid him in his project. In the end, he spent an entire year screening and analyzing almost four thousand papers and processing all the relevant data in a number of databases. This resulted in a useful body of reference for everyone setting out to study the same topic in the future and, most importantly, it enabled him to carry out experiments in a much more focused way. The databases allowed him to filter for different criteria, giving him a quick insight into what had and hadn’t worked in previous studies.
Cultivating cells takes time and requires patience. You need weeks or even months to cultivate osteoblasts from the stem cells before you can add osteoclasts, which are grown from monocytes, a specific type of white blood cells. After that, it takes a few more weeks before you can see the result. In this case, the desired result was for the osteoclasts to resorb the bone tissue and for the osteoblasts to form new tissue – exactly like it happens in the human bone.
These changes cannot be seen with the naked eye. Remmers first made CT scans of his cultivated bones at different stages and then overlaid them, using a software program to calculate which voxels (3D pixels) were different from before. “I knew exactly what it would look like if it worked, but the question was if it would work.” Naturally, mountains of tension were washed away by relief when after months of meticulous work in the lab the computer showed the exact desired result – the osteoblasts and osteoclasts were both active in the model and were both doing precisely what they were meant to: forming and resorbing the bone, respectively.
Personalized treatment
Remmers’ model is a proof of concept, the first realization of a new model. It demonstrates that it’s possible to cultivate a 3D model of osteoblasts and osteoclasts in a lab, but the model is not yet directly applicable. After further elaboration, it could be used for all kinds of research into osteoblasts and osteoclasts, which would help us better understand the bone.
A more elaborate version of the model could also be used to test drugs. This wouldn’t allow for complete replacement of animal testing, which is currently mandatory for human medication, but it could serve as an intermediary step in this process to bring down the number of animal tests.
Remmers’ favorite application, but also the one with the longest way to go before it could become a reality, is personalized treatment of patients. “Imagine being able to cultivate the 3D model from the cells from a patient, enabling you to see exactly how their cells react to certain drugs in the lab. This would allow us to treat patients with osteoporosis in a much more focused way, with drugs we already know to be effective.”


Discussion