Tamer of immune cells
Read more- Research
- 07/11/2018
Tamer of immune cells
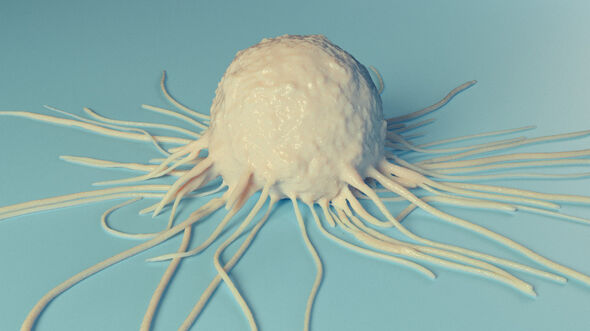
Our bodies are pretty good at healing themselves, only sometimes a helping hand is welcome. This in a nutshell is the thinking behind immune therapy. Immunologist Jurjen Tel is putting his money on the dendritic cell, the surveillance officer of our immune system. With the right training, these cells can be taught to dispatch killer T-cells to fight tumor cells - a last resort against advanced forms of cancer.
Jurjen Tel bears an uncanny resemblance to his beloved dendritic cell, having tentacles extending to other groups within the Department of Biomedical Engineering. As their supervisors, the three PhD candidates in his group Immunoengineering have professors from three different research groups: Carlijn Bouten (Soft Tissue Engineering & Mechanobiology, under which Tel's own team officially falls), Menno Prins (Molecular Biosensing for Medical Diagnostics) and Jan van Hest (Bio-Organic Chemistry).
Leeuwarden
Bringing with him a Veni grant, the successful young researcher - who last summer added a prestigious ERC Starting Grant to his achievements - transferred two years ago from Nijmegen, where he was a postdoc in the group run by Spinoza laureate Carl Figdor. Despite all this, he started out modestly at the higher laboratory school (HLO) in his home city of Leeuwarden, the Frisian reveals after greeting your reporter with a heartfelt firm handshake.
“For my HLO graduation internship I worked in Nijmegen, and that's where I came cross Figdor's group. He is an authority in the field of tumor immunology in Europe, and I was greatly inspired by his work. But, of course, if I wanted to pursue a career in research, I needed to have a Master's under my belt.”
So Tel made his way to the north, to gain a Master's in Medical Biology in Groningen. He did his graduation research in Leiden and meanwhile applied to Figdor in Nijmegen. “Luckily they had a position available, and were even prepared to wait until I had finished my degree.”
We presented dendritic cells with pieces of tumor, thus enabling the immune system to recognize that particular tumor
Tracking
In his doctoral research he concentrated on what is called the plasmacytoid dendritic cell. There aren't many of these immune cells in our body, he explains. “They originate in the bone marrow and end up via the bloodstream in all kinds of tissue, where they detect antigens.”
Antigens are molecules from, say, viruses or bacteria - but sometimes from the body's own cells, like tumor cells - that activate our immune system. “The dendritic cells track down these antigens, themselves getting activated in the process, and present the antigens to so-called killer T-cells, which then attack the pathogens.”
Training
Tel studied how exactly these plasmacytoid dendritic cells do this. “We were the first in the world to succeed in isolating these cells from patients and subsequently activate them, so that after being replaced in the patient they started to secrete what is known as type-1 interferons, proteins that activate the immune system. In addition, we presented the dendritic cells with pieces of tumor, thus enabling the immune system to recognize that particular tumor.” When the dendritic cells are subsequently inside the lymph nodes they encounter the killer T-cells mentioned above. “From the lymph nodes, the killer T-cells go back into the body, in search of the tumor.”
Article continues below the picture.
The major advantage of this kind of immune-based treatment is that the killer T-cells are capable of attacking the tumor cells without harming healthy cells - which is the case with chemotherapy and regrettably still causes severe side effects.
The first study involving the ‘trained’ dendritic cells, conducted on people with metastized melanoma, a highly aggressive form of skin cancer, was so promising that health insurers even covered a follow-up study from the basic insurance package. “That is pretty unique, and it feels really special to have been in on the ground floor of this development - although I am no longer directly involved.”
Artificial
In the meantime, still collaborating with his former colleagues in Nijmegen, Tel is investigating whether it might be possible to produce his favorite dendritic cells artificially. “This is chiefly about something called the antigen-presenting characteristic of dendritic cells: they must be able to point killer T-cells in the direction of the tumor, or in other cases to a virus or bacterium.”
An artificial cell like this would be much cheaper than a vaccine custom-made for each patient - based on the individual's own immune cells. “The pharmaceutical industry is less interested in these forms of personalized medicine; they are simply much too expensive. They would rather have a cupboard full of immune cells suitable for anyone.”
Individual cells
In Eindhoven Tel is now focusing mainly on the subject of his Veni grant: fundamental research on immune cells with the aid of single-cell technology. He explains why it is important to look at one cell at a time. “When we try to find out which dendritic cells are suitable for activating T-cells, we measure hundreds of thousands of them at the same time – the bulk, as we call it. But this kind of average effect probably hides the existence of two or multiple populations of dendritic cells that behave differently.” If that is indeed the case, then you want the ability to select the right cells at the outset, the researcher continues. “That is much more efficient.”
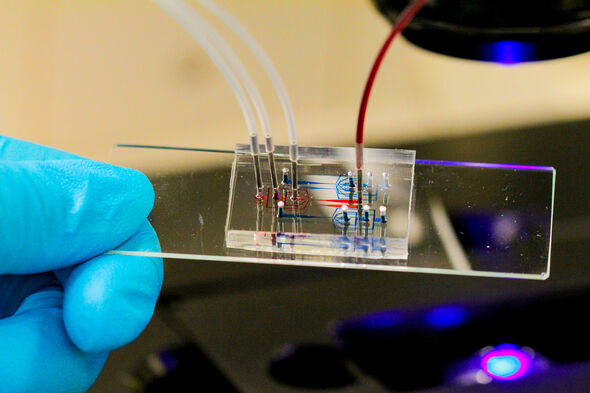
In order to view the immune cells individually, Tel uses microfluidics: a set of techniques whereby a fluid is guided through tiny channels, and which also forms the basis of lab-on-a-chip technology. “I'm collaborating on the work, among others with Tom de Greef here at Biomedical Engineering, and the Microsystems group run by Jaap den Toonder at Mechanical Engineering.”
Droplets
He shows a short film (see above) that shows how the cells in individual droplets are captured by very carefully mixing water, olie and surfactant (a soapy substance). “Each droplet of about 80 picoliter then contains a single cell, and you can stack up these droplets and study them individually for days on end. This also enables you to track the development of individual cells over time.”
One thing at least is evident from these experiments: that solitary dendritic cells behave unlike cells in bulk. “We are managing to activate only 1 percent of the isolated cells; for bulk that figure is roughly 40 percent. It would appear that interaction between the cells is necessary to ensure optimal activation.” Tel wants to use his recently acquired ERC Starting Grant to bring together killer T-cells and dendritic cells in the droplets, to see how they react to each other.
The article continues below the picture.
Chambers
In another experiment, Tel isn't using droplets, but tiny chambers in which the cells can be confined. “We can flow a tiny amount of fluid in there to expose the cells for a set time to certain substances. This is a little more realistic than the tiny droplets. We are currently hard at work getting this system running smoothly.”
While the career of the immuno-engineer is soaring, the limits of his ambitions are still far from being reached. “I cherish a private wish to set up a fully fledged Master's track in immunoengineering. At any rate, I am going to collaborate with Hans Wyss and Jaap den Toonder at Mechanical Engineering to develop the course 'Microfluidicsput to work'; it's the course I tell students interested in my field to take. It was getting full up, but it's essential preparation for students who want to come and work for me. And I could certainly do with a number of them.”


Discussion