CursorOnTour@CE&C | Membranes for a sustainable future
New technologies for making and storing clean energy can help us meet our climate goals, as can ways of capturing CO2 and recovering valuable substances from waste streams. Membranes can play a large part in all these developments, so believe Kitty Nijmeijer and her colleagues at the recently established research group MM/P. “The young people now graduating with us are the engineers who will shape the circular economy and the energy transition.”
A membrane is a filter that allows some substances to pass through but not others. Membranes have been in widespread use for decades, in water treatment plans for example. For some years now the Department of Chemical Engineering and Chemistry has had a research group, Membrane Materials and Processes (MM/P), focused solely on the making, characterization (study) and application of membranes.
MM/P was set up by professor Kitty Nijmeijer and her colleague Zandrie Borneman, both of whom came to TU/e from Twente in 2016 in order to develop a brand new lab on the ground floor of the Helix building. In the MM/P lab researchers are working on applications that are a fair bit more sophisticated than the filtering of waste from water. The membrane experts have been busy since their arrival; anyone looking at the MM/P website today will see some fifteen research projects in which membranes are used for a varied range of applications, such as energy storage and production, the capture of CO2, and the recovery of valuable minerals from waste water.
Perfect membrane
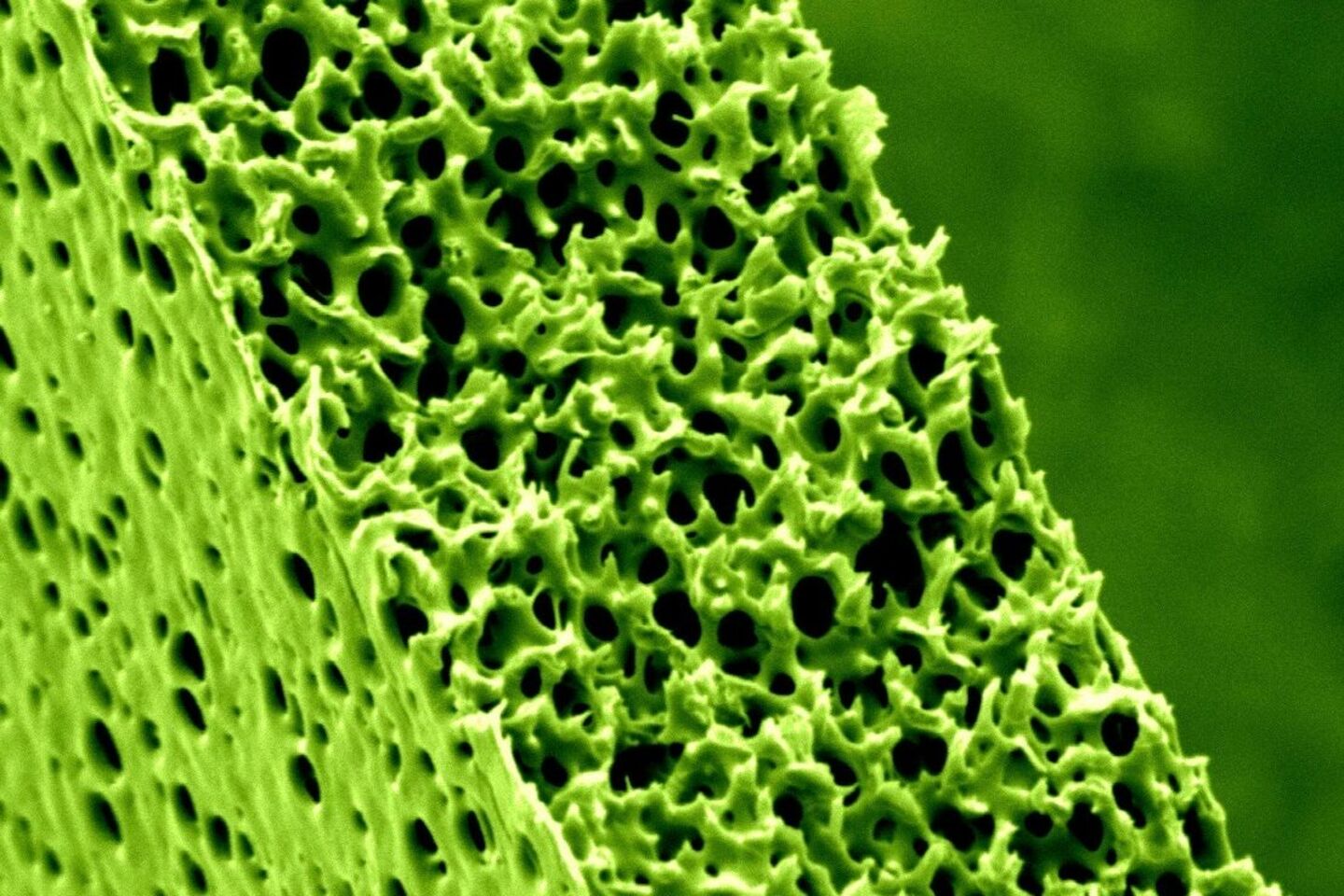
Hanging on the walls of the lab and prominently displayed in Nijmeijer's office are photographs showing microscope images of the membranes the group has made in the lab using polymers (plastic). Their filter function depends not only on the size of the pores but also very much on the chemical properties of the polymer. Among other things, these properties determine the attraction and repulsion between the membrane and the various substances; this interaction also poses one of the biggest challenges facing the researchers since a membrane that becomes fouled too quickly or actually clogs up is useless in the field.
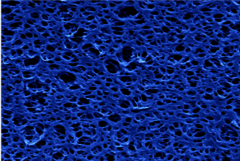
The pores shown on the brightly colored images vary quite a bit in terms of size and orientation, the professor points out. “Ideally, you want to have a perfect membrane, every pore of which is exactly the same size, so that filtration is highly selective,” she says. “We are now trying to achieve this in cooperation with the groups run by Albert Schenning and Rint Sijbesma, with which we are sharing four doctoral candidates.” This collaboration came about after the chemistry professors in question walked past the MM/P's display cabinets in which Nijmeijer has displayed a wide range of membranes - mostly currently available, commercial ones.
“They were curious to know what exactly they were looking at, and one thing led to another,” she relates with a smile. Drawing on the expertise of Schenning and Sijbesma, she believes, it should be possible to make the ‘perfect’ membrane. “They know everything about the chemistry involved while that's an area I am less familiar with, being as I am a chemical engineer. What's nice about this department is that a collaboration like this can come about very easily. There are no internal boundaries, and you can decide for yourself who you work with. We don't need to bill each other for our services.”
In the video below Kitty Nijmeijer shows us around the MM/P lab and uses a demonstration model to explain exactly how the blue energy technology works.
Blue energy
Another striking project on which Nijmeijer and her colleagues are working is Blue Energy. This involves generating electricity by bringing river water and salt seawater into contact with one another through a membrane, she explains. “In this case the membrane doesn't allow any water to pass through, only the salt ions that are inclined to diffuse from the salt water to the fresh water.” And this movement of electrically charged ions creates an electrical current. ‘Blue Energy’ power plants on the boundary between fresh and salt water (at the mouths of rivers, for example) could potentially supply more than 10 percent of electricity demand worldwide, says Nijmeijer.
For some years now a small ‘blue’ power station has stood on the Afsluitdijk (in 2016 this power station was proclaimed a National Icon), and now there are plans for another one, ten times bigger, at Katwijk, which will have a power output of 1 megawatt. “That is enough power for about 1600 households. It is going to cost eighty to a hundred million euros; if the money is forthcoming, construction could start next year.”
Nijmeijer and her colleagues have been involved in blue energy research since its inception. “We are now on our fourth generation of doctoral candidates. Together with Wetsus and a number of companies, we spent the early years doing fundamental research on the membranes needed, and at some point the pilot power station on the Afsluitdijk was realized by Wetsus spin-off REDstack and Fujifilm. With our input, Fujifilm produced the membranes for the large plant while at the same time we were able to test new membranes in a small experimental setup on the Afsluitdijk.”
A field test of this kind is essential if you are working with natural water, emphasizes Nijmeijer. “For example, fouling is a major problem in the field, and you can't simulate that in laboratory water. Just two weeks after we started everything was clogged up with a mass of algae. Filters with special barbs had to be installed so that mussels could not grow on them. And the salt in seawater is not only sodium chloride but also magnesium sulfate, which proved to have a highly adverse effect on the generation of electricity.”
Capture and store
Another research line with potential for helping to solve the climate problem is focusing on the capture of CO2, in power plants for example. This involves using membranes to separate greenhouse gases from harmless gases such as nitrogen and oxygen, Nijmeijer explains. “We are cooperating on this with DIFFER. There, they are converting the CO2 to CO, which is in turn a major raw material for the chemical industry. That's another really great collaboration that's happening here on the campus.”
Energy storage is also an area in which membranes could play an important role in the future. Thus, at MM/P they are working with the group run by departmental colleague René Janssen on what are known as ‘flow batteries’, in which - as in blue energy - membranes are used that allow only molecules with a certain charge to pass through. The idea is that in the future sustainably generated electricity can be cheaply stored in membrane batteries like these. Nijmeijer expects to see this applied initially to small-scale storage, and that the large-scale storage of energy using this technology won't be developed until some years later.
“In this area we are working mainly with Elestor, a company in Arnhem, and they now have a modular system suitable for storing energy from, say, a wind turbine or a roof full of solar panels. But as yet these are still relatively large plants, so we are working hard to make the process more efficient and to increase capacity, so that the plants can be made smaller.”
Circular future
Nonetheless, the membrane expert is optimistic about the future. “In thirty years' time our economy to supposed to be entirely circular. That seems like a huge undertaking, but when you consider that today's industry has been built in less than a hundred years, and much of it since the Second World War, then at the current pace thirty years is quite a long time. The young people now graduating with us will only be their fifties thirty years from now, and they will play an important role in this development. They are the engineers who will shape the circular economy and the energy transition. They'll simply get it done, and we are going to watch it all happen!”
This article is part of the special CursorOnTour@CE&C series, with on-site reporting, this time from the department of Chemical Engineering and Chemistry.




Discussion