Home Stretch | Medicine in a virus
People who suffer from a severe metabolic disorder often have no choice but to make dozens of hospital visits a year to be hooked up to a very costly intravenous drip. To make this treatment more efficient, the medicine could be administered in the protein shell of a virus. Doctoral candidate Daan Vervoort modified the casing to make this possible.
After a solid year of corona pandemic the word ‘virus’ may well send a shiver down the spine of many readers but, as Daan Vervoort points out, these unpleasant pathogens can also be very useful. This at least is the line of inquiry taken in the doctoral project of this biomedical engineer. “The thinking is that you can get medicines to the right spot in the body much more efficiently if you lock them up in the empty shell of virus particles.”
This method can be of great value to people suffering from what are known as lysosomal storage disorders. In these inherited disorders, including Fabry, Gaucher and Pompe disease, waste products accumulate in the body because the patient produces too few enzymes -‘molecular scissors’ - to degrade these substances. This causes all kinds of severe symptoms, precisely which depends on which enzyme is lacking.
Intravenous drip
The most common treatment of storage disorders is to administer the missing enzymes, Vervoort explains. “Which means the patient has to visit the hospital, say, every two weeks to be put on an intravenous drip, which is enough of a burden in itself. Then consider that the vast majority of the medicine doesn't reach the right cells in the body, but is broken down before it leaves the bloodstream.”
Consequently, the therapy is not as efficient as it could be, while producing the enzymes is a costly business. A lifelong treatment of this nature costs about two hundred thousand euros per patient per year, the doctoral candidate says. “Our primary aim is to develop a more effective therapy for these patients, but if the medication can be administered in a very targeted way inside virus particles, you will also need a smaller quantity of enzymes. The cost saving this will bring could make this therapy a good deal more accessible worldwide.”
Text continues below illustration
Over the course of millions of years, viruses have ‘learned’ how to penetrate the cells of their host in order to avail themselves of the cell's replication service. An important part of this evolutionary success depends on the virus's protein shell, which not only protects the viral genetic material, but also ensures that the virus particle can attach to the right host cells. This makes this shell an ideal candidate for delivering medicinal enzymes in a highly targeted manner.
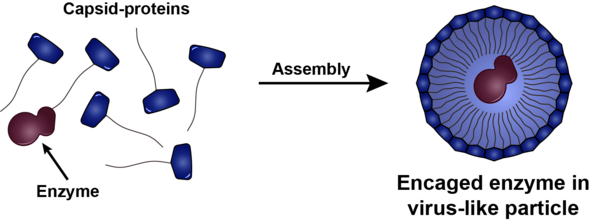
And so, in the Bio-Organic Chemistry research group they are working on casings based on the protein shell of a virus that is found in bean plants. “The big advantage of this particular protein shell is that it spontaneously forms when you bring together under the right conditions its building blocks: sixty identical proteins per shell.” This offers the possibility of locking medicinal enzymes into these protein casings, simply by mixing together the enzymes and the building blocks and then manipulating the temperature, acidity or salinity of this solution.
Blood
Unfortunately, the original protein shells of the bean virus did just the opposite and fell apart at a neutral acidity and a temperature of 37 degrees, the conditions in our blood. So to make the casings suitable for administering medicines, the chemical structure of the building blocks had to be slightly modified. “In our group we have succeeded in producing building blocks that at 4 degrees Celsius do not assemble to form capsids, as we call these casings. This is good, because at this temperature both the enzymes and the capsid proteins stay well preserved. Next, we can form protein shells around the medicinal enzymes, shells that remain stable under physiological conditions. To achieve this, we ‘pinned’ a particular percentage of capsid proteins to the enzymes.”
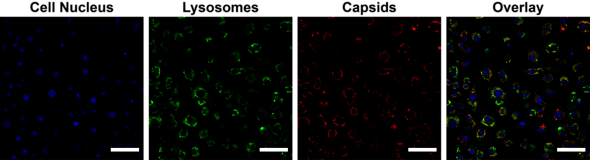
The next step was to demonstrate that the parcels of medicine are actually taken up by the body's cells. By adding a luminous molecule to the casings, Vervoort could see under a fluorescence microscope that the capsids do indeed arrive at the right place in the cell - the lysosomes, where the waste processing takes place. “And I was able to show that the enzymes inside the capsid were still intact,” he explains.
Text continues below image
The researchers now want to see whether the medicine delivery also works in cells already damaged by the storage disorders. “To do this, we will be collaborating with Leiden University, which is growing special patient cells for this purpose.” The road to an actual medicine is still long, it must be said, Vervoort notes. “If this approach is proven to work in the cell culture, we must first find out whether any toxins have been created during the production of the casings before we can consider an animal study. Only then would the first patient studies look likely.”
As for himself, he knows that once he has his doctorate under his belt he will continue his work at Bio-Organic Chemistry, as a postdoc. “I want to research whether protein casings other than virus particles may potentially be suitable for delivering medication to treat lysosomal storage disorders.”


Discussion