Home Stretch | New medicinal key to the back door
When something is awry with your immune system, your digestion or your endocrine systems, nuclear receptors, as they are called, may well be involved. If need be, the operation of these regulator proteins can be altered with medicinal drugs, but this carries the very real risk of unpleasant side effects. Doctoral candidate Femke Meijer looked for - and found - molecules that might well be used as medications for autoimmune diseases, but with fewer side effects.
Our body has exactly 48 types of nuclear receptor. These are proteins that float about in our cells and can be activated by all sorts of signal molecules such as hormones. When this happens, the nuclear receptor in question issues an instruction in the cell nucleus to produce certain other proteins. Shutting down or conversely activating these nuclear receptors is the mechanism by which one in six medicines achieves its intended effect. The best-known example is likely the contraceptive pill, tells doctoral candidate Femke Meijer. “This acts on the estrogen and progesterone receptors.”
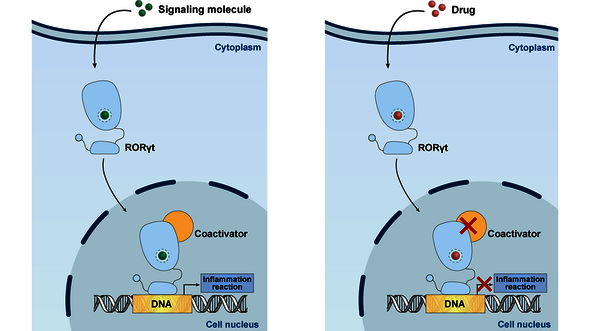
She herself has studied another nuclear receptor, RORỿt, which regulates the production of cytokines and as such plays a role in the genesis of inflammatory reactions. Certain drugs for autoimmune diseases, such as rheumatism, psoriasis, asthma and Crohn's disease, turn this function to their advantage and aim to shut down this nuclear receptor, she explains. “They do this by blocking what's known as its binding site with a molecule, so that this particular nuclear receptor, RORỿt, is deactivated.”
Side effects
Unfortunately the binding sites of all 48 nuclear receptors are fairly similar. This type of drug carries the risk, therefore, of inadvertently affecting other nuclear receptors with very different functions. As Meijer is keen to emphasize, this can lead to unwanted side effects. For example, weight gain in the case of prednisone - which combats inflammatory reactions - because this drug also affects our metabolism. And just think of the nuclear receptors on which the contraceptive pill acts; you wouldn't want a drug for rheumatism to leave you infertile.
In 2015 in the Chemical Biology group led by Professor Luc Brunsveld, which is where Meijer conducted her doctoral research, RORỿt was discovered to have a special property: as well as the usual binding site - the ‘front door’- this nuclear receptor also possesses a ‘back door’ to which other molecules can bind. Marcel Scheepstra, then a doctoral candidate, found a molecule that always blocked the effect of RORỿt, regardless of whether natural hormones were present - as a rule, in high concentrations, hormones reduce the effectiveness of drugs that work in this way.
A promising result then, especially since this molecule proved to have no impact on almost all other nuclear receptors. Evidently because the newly discovered molecule - let's call it a ‘key’ - did not fit the regular binding site, whereas it did fit the back door, and this door, as far as anyone knows, is unique to RORỿt.
Top 100
Femke Meijer's work builds on this result. Firstly, it makes sense to have another key to the back door in reserve as a potential medicine. Secondly, the back-door key found by Scheepstra had two drawbacks, she explains. “That molecule was quickly broken down in the body. What's more, it proved capable of binding to one other nuclear receptor, at its regular binding site.”
And so she trawled through a computer database of some hundred thousand molecules, in search of a ‘key’ with the right shape and chemical properties to fit the RORỿt's back door. “In the top 100 this produced, we kept seeing a certain type of molecule crop up. In the lab we produced a number of versions of this molecule and introduced RORỿt to see whether they would bind.”
A number of steps later, involving repeated tweaking of the new versions, the binding was just about working, but it was still not very strong. “On a positive note, however, we were seeing the candidate molecules arrive at the right place in the protein. At this point we replaced one nitrogen atom with an oxygen atom.” This proved to be the right choice. “The optimized molecule binds much more securely to RORỿt and only to RORỿt; it binds less well to the 'wrong' nuclear receptor.”
Medicine
With this second key to the back door, Meijer believes she has found an interesting prospect for a new drug for autoimmune diseases, one expected to produce fewer side effects. What's more, a drug that uses the nuclear receptor's ‘back door’ could even prove more effective, because it doesn't have to compete with the body's hormones - which, after all, always enter by the ‘front door’.
But, she warns, a medicinal product is still a long way off. “While we have proven the molecule's effectiveness in cells, we have yet to prove the same with living organisms, such as animals or people. Though this work is already occupying my colleagues.” As for herself, Meijer will be bidding the university farewell after her PhD conferral ceremony and will start work at Symeres in Nijmegen, a company doing pharmaceutical research. “There I'm going to be able to do the same type of research as at TU/e, but then closer to the application. 'For real' as it were. I'm excited by the prospect.”


Discussion