Home Stretch | Cellular pushing and pulling
Roel Kooi developed a new system with magnetic cilia
The cells in our body are constantly exposed to a wide range of forces – cells pulling on each other, flowing fluids exerting pressure, the effects of actions like jumping or simply the pull of gravity. TU/e researcher Roel Kooi developed a new system with magnetic cilia – tiny vibrating hairs – to grow cells in a culture dish “under stress”. On Tuesday, he will defend his dissertation at the Department of Mechanical Engineering.
The athletes among us know it well: when you subject your body to mechanical forces, your muscles grow and your bones get stronger. Movement scientists describe the impact of these forces at the body level, but a lot happens at the cellular level as well. And that is what PhD candidate Roel Kooi focuses on. Four years ago, he left Wageningen for a challenging PhD track at TU/e in the Microsystems research group. There, he worked on directing bone cells using magnetic cilia. “Mechanical forces cause cells to send all kinds of signals and change their behavior. That’s ultimately what allows your muscles and bones to grow.”
Mechanical stress isn’t just important for growing cells – it’s essential for any cell to function properly, Kooi emphasizes. “You can see this very clearly in the bodies of bedridden patients, where a lot changes at the cellular level due to lack of movement. Or in astronauts who spend long periods of time in outer space, where their cells are no longer exposed to gravity. Their hearts pump less forcefully, and their other muscles also gradually atrophy. It’s a downward spiral.”
Replacing diseased kidney cells
In recent years, we’ve gained more and more insight into what forces affect cells in the body and how. But replicating them, that’s a different story. Still, it’s important, Kooi explains. “Of course, you want cells to grow in the most realistic way possible when you’re using them in your research. Mechanical stress causes cells to produce different proteins and behave differently as a result. And that, in turn, causes them to send out different signal substances to their surroundings.”
In addition, Kooi and his colleagues want to explore how to influence cell behavior itself. “For example, when you’re trying to stimulate a tissue to repair itself. Or if you want to direct the differentiation of stem cells into specific types of body cells, such as bone, cardiac muscle or kidney cells. For these kinds of biomedical applications, we’re looking for a method that allows us to control cells as precisely as possible.”
In Professor Jaap den Toonder’s research group, many experiments are conducted with magnetic “cilia”. With these tiny artificial vibrating hairs, Den Toonder developed a new technology to control forces and flows in mini-labs, such as an Organ-on-a-Chip. Until now, it had not been possible to grow cells on a substrate of magnetic cilia and stimulate them mechanically. And it proved to be quite a challenge, Kooi says with a laugh.
Cilia forest
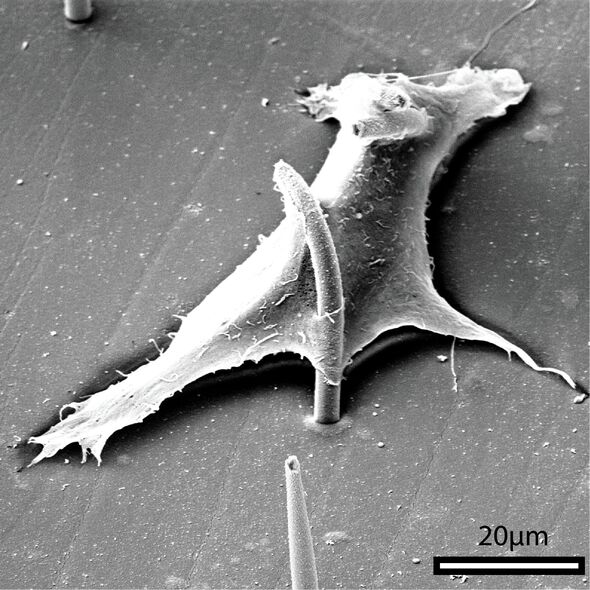
“The cilia are made from a flexible magnetic polymer. We can create pillars that are 6 micrometers long and 400 nanometers in diameter, but the most commonly used ones are 23 micrometers in length and 2 micrometers in diameter. That’s about 30 times thinner than a human hair, which makes them well-proportioned for our cultured cells. The pillars shouldn’t be too large, because we want the cells to grow between the cilia, not on top of them. And the density must be right too; otherwise, it turns into a chaotic cilia forest.”
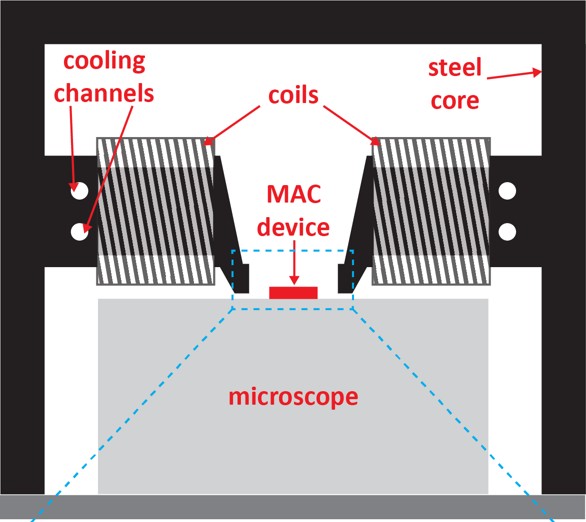
This platform, along with an electromagnet, formed the basis for a new setup Kooi developed. “In this lab system – NanoMAC (Nano Magnetic Artificial Cilia) – we can now control the cilia with extreme precision. How fast they move, with how much force, and for how long.”
The cells turned out to grow well on the polymer substrate – a promising result. But to his frustration, Kooi found that, with the cells included in the setup, the cilia stopped moving altogether. “That cost quite a bit of time and head-scratching. Eventually, we figured out it was due to the fluid environment. We could control the cilia’s movement very precisely in ethanol, but cells need an aqueous environment. And that proved detrimental to the cilia’s mobility.”
Minuscule magic
With a modified hydrogel substrate, it finally worked. So now, for the first time, cells can be cultured with precise control thanks to steerable magnetic cilia. Under the microscope, Kooi was able to study the interaction between the cells and the cilia in detail, he says enthusiastically. “We can actually grow the cells in between the pillars. And at the same time, we worked on a microscope setup that allows us to live monitor the cells as we move the cilia. It took some puzzling to get the electromagnet, mini-lab and visible cells all under the microscope at the same time.”
So the proof of concept is in place. Now, Kooi hopes a new PhD candidate can continue with the optimization and application. Other cell types – Kooi used bone cells as a model –, other forces and interactions. “This technique offers a lot of possibilities. For more realistic cell culture studies, and to take another step towards regenerative therapies. And then we can also start integrating it with the Organ-on-a-Chip to gain insight into disease development and drug efficacy testing. These minuscule – almost magical – cilia are opening up more and more possibilities.”
PhD in the picture
What is that on the cover of your dissertation?
An artistic representation of the platform with magnetic cilia. We always view it from above, but here, you see the pillars from the perspective of the cell. Kind of like a cilia forest.”
You’re at a birthday party. How do you explain your research in one sentence?
“I try to create a very detailed picture of how a cell really behaves, and how we can control that from the outside.”
How do you blow off steam outside of your research?
“Quite literally. I’ve been playing the saxophone since I was nine, and jazz is really my thing. Music takes your mind off things and it can be very meditative. I also play in a few bands, and I’ve found that music really brings people together; it’s a fun, social experience.”
What tip would you have liked to receive as a beginning PhD candidate?
“Read a lot, ask a lot of questions. When I first started, I spent a lot of time figuring out how to go about my project. You don’t have the most efficient solutions right away. So keep in mind that you don’t have to reinvent the wheel.”
What is your next chapter?
“After eleven years at the university, I’d like to explore opportunities outside the world of academia. Preferably within my own field, though.”


Discussion